The Cohen Lab is investigating a diverse array of questions related to adipose biology and metabolic disorders associated with obesity, including hypertension, diabetes, dyslipidemia, and many types of cancer. One in three adults in the US are obese, leading to nationwide health care costs upwards of $100 billion per year. We are committed to uncovering the molecular mechanisms driving metabolic dysregulation in obese individuals, which may point the way towards novel therapies.
Adipose function is influenced by both its location and constituent cell types. For instance, obese individuals with increased visceral fat experience low-grade chronic inflammation and are much more likely to develop co-morbid conditions, whereas obese individuals with increased subcutaneous fat are less likely to develop such sequelae. If we probe further down to the cellular level, we find that there are several different types of fat cells, or adipocytes. White adipocytes store energy in lipid droplets and can contribute to inflammation in the setting of obesity. Brown adipocytes are rich in mitochondria and can dissipate energy through adaptive thermogenesis, with anti-obesity and anti-diabetic effects. Beige adipocytes are embedded within adult white adipose depots and can be activated by cold, sympathetic input, or other stimuli to dissipate energy, similar to brown adipocytes. Prior work from our lab has shown that the phenotype of fat cells is regulated by a transcriptional coregulatory protein called PRDM16, which when deleted results in an ablation of beige fat and increased unhealthy visceral-like fat. We are now focused on further dissecting the key molecular controls governing fat cell phenotype with the ultimate goal of engineering healthier fat.
Adipose Tissue Microenvironment
Co-developed with Marc Tessier-Levine’s lab, we adapted the iDISCO whole-tissue clearing protocol to suit the unique optical properties of adipose tissue. We have applied this technique initially to study the interaction between the sympathetic nervous system (SNS) and adipocytes and discovered that, in addition to the well-described regulatory role which SNS fibers exert on adipocytes, adipocytes themselves can also regulate the patterning of SNS in adipose tissue. We are now applying this technique to a range of questions relating to the adipose tissue microenvironment.
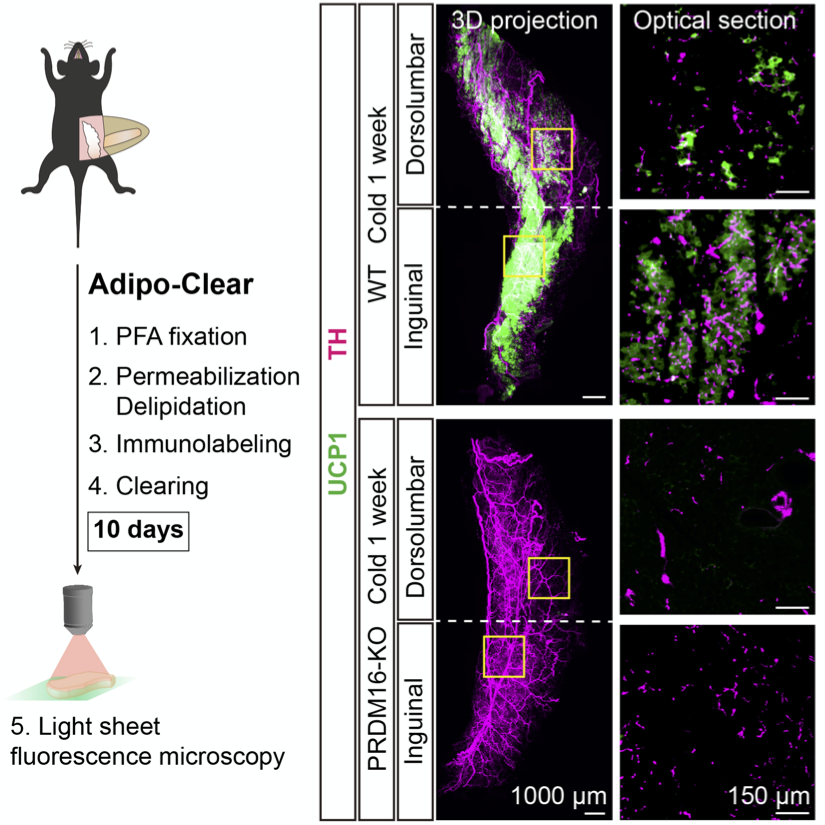
Inter-Organ Communication
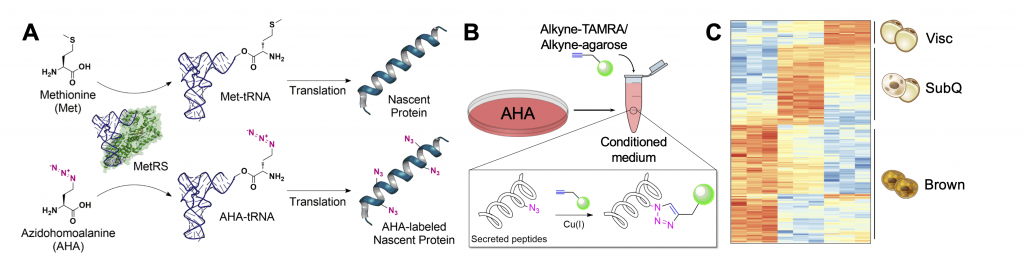
Since the discovery of leptin, adiponectin, and adipsin in the 1990s, adipose tissue has been appreciated as a key endocrine organ regulating diverse physiological processes. These include feeding behavior, thermogenesis, insulin sensitivity, insulin secretion, angiogenesis, and more. Bioinformatics analyses suggest that as many as 1000 proteins may be secreted from adipocytes. We are interested in studying the secretome of adipose tissue, as well as questions around inter-organ communication more broadly in health and disease. Recently, we have employed bio-orthogonal non-canonical amino acid tagging (BONCAT) to selectively label and enrich for polypeptides secreted by fat. Several projects in the lab are involved in scaling and optimizing this approach for adipose tissue and other organ systems in diverse biological contexts.
Obesity and Cancer
Obesity is a known risk factor for many diseases. Increasingly, obesity is being linked to increased morbidity and mortality in cancer, as well as increased incidence of various cancer types. Obesity has now eclipsed smoking as the leading preventable cause of cancer. However, the mechanistic links between obesity and cancer initiation, progression, and outcome have been understudied. We have recently discovered that adipocyte-derived creatine acts as a fuel for a model of triple-negative breast cancer. We are interested in examining the link between obesity and cancer in other cancer types and whether adipocyte-derived creatine may act as a fuel in other cancer types.
Brown Fat in Humans
In 2009, functional brown fat was identified in adult humans through 18F-FDG-PET/CT scans using radiolabelled glucose. This generated hope for those hoping to employ brown fat biology in the treatment of human metabolic dysfunction. Recently, our lab extended these findings and showed in a retrospective cohort of 53,475 patients who received 18F-FDG-PET/CT scans, patients who had detectable brown fat on their scan had lower odds ratios of a host of factors, including type II diabetes, coronary heart disease, and hypertension. The lab is broadly interested in translational research to understand the role of adipose tissue in health and disease.